The Difference Between Propylene Glycol and 1,3-Propanediol

introduction:
In the sophisticated world of organic chemistry, the differences between isomers often contain profound scientific principles. Propylene Glycol and 1,3-Propanediol, both isomers, have the same molecular formula of C3H8O2, but show completely different arrangements in the spatial distribution of hydroxyl groups.
This seemingly minor structural difference actually leads to significant differences in a series of properties such as molecular polarity, hydrogen bond network, and thermodynamic behavior, which in turn shapes very different industrial application scenarios. From the electron cloud distribution of molecular orbitals to the mechanical properties of macroscopic materials, from biological metabolic pathways to environmental degradation behavior, this comparative study of isomers provides an excellent model for understanding structure-property relationships.
Propylene Glycol usually refers to 1,2-Propylene Glycol, whose two hydroxyl groups are located on the first and second carbon atoms of the carbon chain, respectively. This structure gives Propylene Glycol a certain uniqueness in spatial arrangement, which in turn affects its interactions with other substances.1,3-Propanediol contains two hydroxyl groups located on its first and third carbon atoms, respectively, creating an arrangement which greatly affects their physical and chemical properties as well as application fields. In its molecular structure, this has an impactful influence on polarity as well as hydrogen bonding abilities between molecules which create differences in solubility, boiling point, etc between substances.
Molecular structure differences of Propylene Glycol and 1, 3-propanediol
The spatial arrangement of hydroxyl groups plays a key role in defining the electronic structure characteristics of molecules. For instance, in 1,2-Propylene Glycol two hydroxyl groups occupy adjacent carbon atoms which causes significant rearrangement of its molecular orbital. Quantum chemical calculations demonstrate that adjacent hydroxyl groups form a conjugated system with their single electron pairs forming conjugated bonds, leading to redistribution of electron cloud density on oxygen atoms. This electronic effect not only increases the acidity of 1,2-Propylene Glycol, which has a pKa value of 14.6, which is more acidic than 1,3-Propanediol at 15.3 but also interferes with its coordination ability for metal ions. 1. 3-Propanediol stands out with its evenly dispersed hydroxyl groups at both ends of its carbon chain, creating an orbital that presents more localized features that give this chemical substance unique spatial selectivity for catalytic reactions.
How many intramolecular hydrogen bonds form is directly tied to the microstructure of a solution. 1.2-Propylene Glycol forms an impressive five-membered ring structure thanks to hydrogen bonds between adjacent hydroxyl groups that form stable intramolecular hydrogen bonds that significantly decrease its polarity in solution. Raman spectroscopy analysis indicates that approximately 68% of 1,2-Propylene Glycol molecules contain intramolecular hydrogen bonds in their liquid state, whereas 32% form intermolecular bonds. Hydrogen bond distribution leads to its low surface tension of about 36mN/m at 25degC, providing it with exceptional spreading agent performance for cosmetic formulations. However, for 1,3-Propanediol due to its large distance between hydroxyl groups being too far apart to form intramolecular hydrogen bonds, more than 90% of hydrogen bonds in its liquid structure form intermolecular interactions and thus lead to its surface tension reaching 42mN/m for greater wetting properties.
Stereochemistry plays an immensely significant role in materials science. 1,2-Polypropylene Glycol’s unique chiral properties make it highly advantageous in the production of optically active materials, particularly liquid crystal materials where its structure can induce orderly mesophase arrangements. 1. 3-Propanediol’s symmetry makes it ideal for creating regular crystal structures, as evidenced by its crystal X-ray diffraction analysis which showed molecules in its crystal exhibit fully crossed conformations, giving synthesized polyester fibers excellent dimensional stability. 1. 2-Polypropylene Glycol may form cyclic intermediates during polymer synthesis, limiting its ability to participate in linear polymerization; on the other hand, 1,3-Propanediol’s terminal hydroxyl group lends itself more readily to producing long-chain polymers – this characteristic makes it particularly valuable in producing polyurethane elastomers.
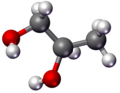
Propylene Glycol
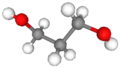
1,3-Propanediol
Property differences of Propylene Glycol and 1, 3-propanediol
The difference in thermodynamic properties is fully reflected in the industrial separation process. Due to the presence of intramolecular hydrogen bonds, the vaporization enthalpy of 1,2-Propylene Glycol is about 15% lower than that of 1,3-Propanediol, which makes the relative volatility of the two significantly different during distillation separation. Dynamic thermomechanical analysis shows that the glass transition temperature (Tg) of polyester materials prepared from 1,3-Propanediol is more than 20 ℃ higher than that of 1,2-Polypropylene Glycol based materials, and this difference is due to the different degrees of rigidity of the molecular chains.In terms of thermal decomposition behavior, thermogravimetric-mass spectrometry analysis revealed that the main decomposition products of 1,2-Propylene Glycol are acrolein and formic acid, while 1,3-Propanediol tends to generate propionaldehyde and formaldehyde. This difference in decomposition path directly affects the thermal stability evaluation of the two.
1,2-Propylene Glycol has a weaker adsorption capacity at the oil-water interface, and its interfacial tension reduction efficiency is only 60% of that of 1,3-Propanediol, which makes the latter more advantageous in emulsion stability. However, due to the presence of intramolecular hydrogen bonds, 1,2-Propylene Glycol maintains a more cyclic configuration at the interface, and this conformation limits the performance of its interfacial activity.
The difference in biocompatibility determines the different fates of the two in the medical field. The metabolic pathway of 1,2-Propylene Glycol is mainly converted into lactate by alcohol dehydrogenase in the liver, which is highly compatible with the endogenous metabolic pathway of the human body. Therefore, it is listed as a recognized safe substance (GRAS) by the FDA.The metabolism of 1,3-Propanediol requires a special diol dehydratase, which is expressed at very low levels in mammals, resulting in a prolonged biological half-life and the possible production of toxic intermediates such as propionaldehyde. Cytotoxicity experiments show that the IC50 value of 1,3-Propanediol for HepG2 cells is about 1/3 of that of 1,2-Propylene Glycol. This toxicity difference limits its application in systems that directly contact organisms.
In terms of environmental fate, the degradation mechanisms of the two substances are in sharp contrast. The aerobic biodegradation of 1,2-Propylene Glycol is mainly carried out through the β-oxidation pathway, and its final products are carbon dioxide and water. The biodegradation rate can reach more than 85% in 28 days. The degradation of 1,3-Propanediol requires a special metabolic pathway. Some strains convert it into β-hydroxypropionic acid through coenzyme B12-dependent dehydratase. This process is slower, resulting in its stronger persistence in the environment. Photolysis experiments showed that 1,2-Propylene Glycol mainly underwent a chain-breaking reaction induced by hydroxyl radical attack under ultraviolet light, while 1,3-Propanediol was more likely to undergo intramolecular dehydration to generate acrolein.
Recent Post

The Difference Between Propylene Glycol and 1,3-Propanediol
How Toxic is Dichloromethane Liquid?

EFFICIENT PROFESSION QUALITY
Focus on professional, is to provide customers with valuable information about Catalysts and additives.
Differences in the synthetic pathway of Propylene Glycol and 1, 3-propanediol
Technological roadblocks on industrial synthesis routes shed light on structural differences that exacerbate bottlenecks. 1.2-Propylene Glycol production using epoxy propane hydration presents one of its greatest challenges – managing its by-products dipropene Glycol and tripropene Glycol through precise control of temperature and pressure of hydrolysis reaction. 1,3-Propanediol can be synthesized using two-step hydrogenation of acrolein, which requires an extremely effective anti poisoning catalyst and requires great care not to deactivate through aldehyde polymerization. Biosynthesis studies of 1,2-Propylene Glycol use the Escherichia coli methylglyoxal pathway while Klebsiella pneumoniae’s glycerol metabolism pathway plays an integral part of its biosynthesis process for 1,3-Propanediol production. There are various metabolic regulation strategies used by both strains.
In the field of materials engineering, the differentiated characteristics of the two diols have given rise to complementary application scenarios. The molecular flexibility and lower viscosity of 1,2-Polypropylene Glycol make it an ideal low-temperature refrigerant, with a freezing point of -59 ℃ much lower than that of 1,3-Propanediol at -28 ℃. This characteristic is irreplaceable in temperature control systems in the aerospace industry. The high boiling point and thermal stability of 1,3-Propanediol give it an advantage in the field of high-temperature heat transfer fluids, especially in solar thermal power generation systems, where its decomposition temperature above 300 ℃ meets the heat transfer requirements of focused solar energy.
The advancement of molecular simulation technology provides a new perspective for understanding the differences between these two substances. Calculations based on density functional theory indicate that the bond energy of intra molecular hydrogen bonds in 1,2-Polypropylene Glycol is approximately 16 kJ/mol, which is equivalent to one-third of the typical hydrogen bond energy. This relatively weak interaction explains the variability of its structure in solution. The intermolecular hydrogen bond network of 1,3-Propanediol shows a stronger synergistic effect. Monte Carlo simulations reveal that there are a large number of branched hydrogen bond clusters in its liquid structure.This unique network structure can be directly tied to its high viscosity characteristics. Simulated molecular dynamics simulations reveal that 1,2-Polypropylene Glycol’s diffusion coefficient is one order of magnitude higher than 1,3-Propanediol due to weaker intermolecular forces and its consequent higher self-diffusion coefficient.
Spectral differences of Propylene Glycol and 1, 3-propanediol
The difference in spectral characterization methods provides a reliable basis for distinguishing the two substances. In the near-infrared spectrum, 1,2-Propylene Glycol shows a unique frequency absorption peak at 1450 nm, which originates from the stretching vibration mode of its intramolecular hydrogen bond. The characteristic absorption of 1,3-Propanediol in this region appears near 1380 nm, corresponding to the deformation vibration of its intermolecular hydrogen bond. In the nuclear magnetic resonance hydrogen spectrum, the chemical shift of the hydroxyl proton of 1,2-Propylene Glycol appears near 1.5 ppm, while that of 1,3-Propanediol appears at 2.1 ppm. This shift difference reflects the significant difference in the chemical environment of the hydroxyl group.
In the future development direction, the differentiated properties of the two Propylene Glycols will continue to promote material innovation. 1,2-Propylene Glycol has broad application prospects in the field of flexible electronics. Its low dielectric constant and good plasticity are suitable for the preparation of stretchable conductor substrates. The potential of 1,3-Propanediol in the field of high-performance fibers is being explored. The modulus of polyester fibers synthesized from it can reach more than 3.5 GPa, which provides new ideas for the upgrading of bulletproof materials. In the field of energy storage, 1,2-Propylene Glycol can effectively improve low-temperature performance as an additive in lithium-ion battery electrolytes, while the application of 1,3-Propanediol in flow batteries shows excellent oxidative stability.

Safety of Propylene Glycol and 1, 3-propanediol
Propylene Glycol and 1,3-Propanediol have low toxicity, but their potential impact on the environment and human health should still be taken into account during production, use, and disposal. In the production process, measures such as sealing and automation should be taken to reduce the contact of operators, and effective treatment of wastewater and exhaust gas should be carried out to ensure that emissions meet standards. At all stages, excessive use and random discharge must be prevented to protect environmental media such as soil and water bodies from pollution. Food, medicine and cosmetic products that come into direct contact with human bodies such as food, medicine and cosmetics require strict dosage and quality standards that must be strictly managed; once discarded they should be recycled or processed according to relevant regulations so as to minimize environmental pollution.
Through in-depth research into Propylene Glycol and 1, 3-Propanediol, we can more efficiently utilize their advantages, mitigate risks, and realize sustainable development. When developing new production processes, emphasis must be given to improving economics and selectivity while decreasing by-product generation; when developing new application fields, all potential environmental and health effects should be thoroughly taken into account; product design/formulation optimization should aim at minimizing usage while improving utilization efficiency; this applies equally for product designs containing Propylene Glycol/ 1, 3-Propanediol when developing new production processes or developing new application fields; when developing new production processes or application fields involving new production processes or application fields containing new by-product generation from production processes as when developing them.
Propylene Glycol and 1, 3-propanediol, two essential chemical raw materials, have an integral place in modern industry and daily life. Their distinct chemical structures, physical properties, chemical properties, production/prep processes and application fields give each material its own special value for production and daily living. As technology progresses and production processes improve further, their uses will broaden even further; further contributing to human production/life.
