Dimethylformamide CAS 68-12-2


High Quality Dimethylformamide CAS 68-12-2 With Good Service
- Appearance:Liquid
- Purity:99.8%
- Delivery:30days
- Sample Available:Available
- Payment:L/C,T/T,D/P,Paypal,Money Gram,Western Union
- Incoterm: FOB,CFR,CIF,EXW,FCA,CPT,CIP
- Transporta:Ocean, Land,Air, DHL,TNT FedEx
Name: Dimethylformamide
CAS: 68-12-2
MOQ: 1KG
Directory Guidance on Dimethylformamide
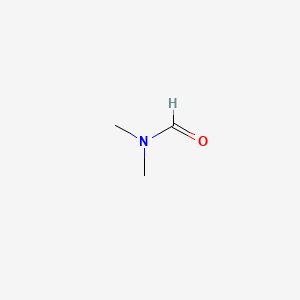
Chemical Structure
Basic Info:
| Melting point | -61 °C (lit.) |
| alpha | 0.94 º |
| Boiling point | 153 °C (lit.) |
| density | 0.944 g/mL (lit.) |
| vapor density | 2.5 (vs air) |
| vapor pressure | 2.7 mm Hg ( 20 °C) |
| refractive index | n20/D 1.430(lit.) |
Product Introduction:
Dimethylformamide (C3H7NO) is an organic compound with important industrial uses. At room temperature, it is colorless and transparent, with ammonia or fishy smell; Industrial grade products may have a more pronounced odor due to trace impurities. In terms of molecular structure, the hydroxyl group of formic acid is replaced by dimethylamine in the molecular structure, forming an unusual spatial configuration-positively charged nitrogen atoms are surrounded by two methyl groups, and negative ions are difficult to access due to steric hindrance. Dimethylformamide is an ideal aprotic polar solvent with stable physical properties. The melting point is -61℃, and the boiling point is as high as 153℃. The density was 0.948 g/cm3 (20℃). It also mixes well with water and most organic solvents. Dimethylformamide has good thermal and air stability at room temperature. But at temperatures above 350 ° C, it breaks down into carbon monoxide and dimethylamine. The production of Dimethylformamide usually involves a direct reaction between formamide and dimethylamine, or the condensation of dimethylamine methanol solution with carbon monoxide catalyzed by sodium alcohol.
Dimethylformamide is an excellent multi-purpose solvent due to its excellent solubility. Its intramolecular exposed anions have high activity and can effectively dissolve molecular materials such as polyethylene, polyvinyl chloride and polyacrylonitrile. It has wide compatibility with inorganic salts and organic compounds. This excellent solubility is due to its unique electron distribution: the strong polarity and dielectric constant (36.7 F/m) provided by its amide groups enhance its solubility; The hydrophobic methyl group enhances its affinity with non-polar substances. Dimethylformamide plays two important roles in chemical reactions; Both acting as an inert medium and reducing the activation energy through its solvation, it reduces the activation energy while also significantly speeding up the ionic reaction. Dimethylformamide can be an ideal carrier for the generation of ester compounds from carboxylate and halogenated hydrocarbons with large steric hindrance, as its selective solubility of isomers allows chemical separation processes involving isophthalic acid and terephthalic acid to meet standards for industrial applications.
From the point of view of chemical stability, Dimethylformamide is highly reliable under standard reaction conditions. The C-N bond in its molecular structure is between single and double bonds, providing protection in acidic or alkaline environments while avoiding side reactions that are prone to occur in highly polar protic solvents. It will hydrolyze under high temperature, strong acid or strong base conditions. In this case, it produces dimethylamine and formic acid as by-products. The properties of Dimethylformamide not only limit its use under extreme conditions, but also provide a controlled decomposition pathway for specific synthetic routes. Dimethylformamide should avoid contact with oxidants during storage and transportation, and pay special attention to ventilation explosion-proof, and the explosion limit of mixing steam with air is 2.2% to 15.2%.
Nature and Specifications:
| Item | Specification |
| Product Name | Dimethylformamide |
| CAS No. | 68-12-2 |
| Appearance | Liquid |
| Shelf Life | 2 years |
| Packing | As your requirements |
| Fp | 136 °F |
| storage temp. | Store at +5°C to +30°C. |
| solubility | water: miscible |
| pka | -0.44±0.70(Predicted) |
| form | liquid |
| color | APHA: ≤15 |
Product service:
- Certificate Of Analysis (COA)
- Material Safety Data Sheet (MSDS)
- Route of synthesis (ROS)
- Method of Aanlysis (MOA)
- Nuclear Magnetic Resonance (NMR)
- Packing pictures and loading video before loading
- Free Sample
- Factory audit
1. Chemical Synthesis Field
Dimethylformamide can be used as an excellent solvent in chemical synthesis reactions. As a polar aprotic solvent with strong solubility, DMF dissolves a variety of organic and inorganic compounds. For instance, organic synthesis reactions require specific solvents, with Dimethylformamide serving as an excellent reaction medium that provides an ideal environment for the reaction while simultaneously increasing contact between reactants and product and improving rates and yield. Key organic reactions that utilize DMF include Friedel-Crafts reaction and Grignard reaction – two important organic reactions where DMF plays an integral part.
2. Polymer Industry Field
Synthetic Fiber Production: Dimethylformamide is an ideal solvent for many polymers such as polyethylene, polyvinyl chloride, polyacrylonitrile and polyamide – including those that make up synthetic fibers such as polyacrylonitrile fibers – making it suitable for wet spinning in wet spinning processes such as polyacrylonitrile fiber production. During wet spinning processes DMF dissolves polymers to form uniform spinning solutions, which are then expelled through spinning nozzles before solidifying in coagulation baths in order to form fibers.
3.Electronic Field
Dimethylformamide’s strong polarity and solubility enable it to effectively remove grease, dirt, and flux residues from electronic components during their manufacturing processes – for instance integrated circuit chips, printed circuit boards etc – so as to improve performance, reliability, and ensure good electrical properties during subsequent assembly and use.
Dimethylformamide can serve both as a solvent and reaction medium in the preparation of electronic materials such as electronic pastes, photoresists and photoresistors, etc. By dissolving solid components to form suitable viscosities and fluidities for coating, printing or other operations. Furthermore, DMF may also be utilized as part of some chemical reactions like electroplating or chemical vapor deposition that take place simultaneously with DMF – either participating directly in them or as an indirect catalyst that influences reaction rate and product performance.
4. Medical Field
Drug Synthesis: Dimethylformamide is often utilized in drug synthesis as both a solvent and reaction medium, offering many different opportunities to synthesize drug molecules in one specific system. Among others, DMF dissolves various intermediates and reaction reagents necessary for reaction reactions; DMF’s favorable environment facilitates antibiotic, analgesic, anticancer drug synthesis by improving efficiency and yield.
5. Pesticide Field
Dimethylformamide Intermediates: Dimethylformamide is an integral component in the production of various pesticides, from dimethylformamide sulfoxide (an insecticide) to DMF used as an active ingredient synthesised from other chemical substances by reacting with chloromethane; all while producing active agents designed to protect crops against diseases and pests.
6. Dye and Coating Field
Dye Synthesis: When synthesizing dye molecules, Dimethylformamide serves both as a solvent and reaction medium during their synthesis process. Many dye synthesis reactions require organic solvents; in these instances Dimethylformamide dissolves intermediates and reaction reagents so as to enable smooth reaction pathways with specific colors and properties of finished products being synthesized from it; it has also proven invaluable when creating disperse or reactive dyes (DMF can play an essential part in their formation). For instance it plays an essential part when synthesizing disperse or reactive dyes etc.
1. Good solubility
Can dissolve a variety of organic and inorganic substances: Dimethylformamide is a highly polar aprotic solvent. Its polar characteristics lie in its ability to interact with a variety of organic and inorganic compounds through hydrogen bonds or other means. For example, it can dissolve polymers such as polyethylene and polyvinyl chloride as well as some insoluble organic compounds and inorganic salts. Therefore, it is a very effective solvent in the fields of synthetic chemicals, processing polymers, and preparing materials, providing convenience for various reactions and processing procedures.
Compatibility with various solvents: Dimethylformamide can be mixed with water and all common organic solvents, including alcohols, ethers, halogenated hydrocarbons, and aromatic hydrocarbons. This excellent miscibility makes the product particularly widely used in the preparation of mixed solvents. Other solvents can be used and mixed in prescribed proportions according to different dissolution requirements and reaction conditions to optimize the dissolution effect and reaction performance.
2. Good chemical stability
Stable under acid-free and alkaline water conditions: Under acid-free and alkaline water conditions, Dimethylformamide can remain stable at higher boiling temperatures, without decomposition or degradation, so it can be used in many organic synthesis reactions, which require a relatively stable environment to ensure the smooth progress of the reaction, avoid solvent decomposition, side reactions caused by reaction selectivity, and the formation of impurities, so as to achieve the purpose of purifying the product.
Can be used as a reaction medium: Since Dimethylformamide is chemically inert and does not react with reactants, it can not only be used as a solvent to dissolve reactants, but also directly participate in certain chemical reactions and act as a reaction medium to affect the reaction mode and product properties. For example, in some organic synthesis reactions, Dimethylformamide can react with reactants to promote the reaction and increase the conversion rate and yield of the reaction. In addition, after the completion of such reactions, it will remain relatively stable, making it easy to separate and recover.
3. High reaction intensity
Tightly solvated intermediates: The positive end of the Dimethylformamide molecule, i.e. the charged end, forms a solvation shell composed of methyl groups, which can act as a spatial barrier to prevent negative ions from approaching, and can only enter the vicinity of positive ions. The activity of naked negative ions is much greater than that of solvated negative ions. Therefore, many ion reactions are easier to carry out in Dimethylformamide than in conventional protic solvents.
For example, the room temperature reaction of carboxylates with halogenated hydrocarbons in DMF is a very good method for synthesizing esters (especially sterically hindered esters) with high yields. This may be a way of choice for DMF to effectively catalyze difficult reactions and make some organic compounds by manipulating specific structures and specific functions.
Contact Us
Product Package picture:



Related References:
chemicalbook-Dimethylformamide
Dimethylformamide Manufacturer
Contact Us
As an experienced Dimethylformamide manufacturer and supplier, Look Chemical is committed to producing and selling high quality products.
We cooperate and trade with 6000+ factories around the world, and our high-quality products and excellent services make us enjoy a high reputation internationally.
As Dimethylformamide CAS 68-12-2 supplier, Look Chemical provides supply chain solutions to partners and customers in a wide range of industries. We offer competitive pricing and quality products.
If you have a demand for this product, please contact our company’s sales staff, we will provide you with a solution in the shortest time.
Transport proposal

1. For products ≤50kg, we recommend using express delivery, which is usually called DDU service (discounted, convenient).
2. For products ≤500kg, we generally recommend air freight, which is usually called FOB, CFR or CIF service (fast and efficient).
3. For products >500kg, we generally recommend shipping by sea, which is usually called FOB, CFR or CIF service (economical, safe).
4. For high-value products, please choose air or express to ensure the safety of product transportation.
Shandong Lookchemical service:
* Timely reply and 24 hours online, the professional team will provide you with the most favorable prices and high-quality products.
* The sample supports testing and inspection.
* Each batch of products will be tested to ensure that its quality meets user needs.
*Packaging can also be made according to customer requirements.
*Any inquiries will be answered by our relevant personnel within 24 hours.
*We will provide you with commercial invoice, packing list, packing list, COA, health certificate and certificate of origin if you need it. If your market has other special requirements, please let us know.
*We will monitor the logistics information in real time and will share the information with you.
* You can consult us at any time if you have any questions about the product, and we will answer you in time.
*If you have any questions about the product, you can report it to us, we will deal with it in time for you, and the product can be returned.
Contact Us
Frequently Asked Questions(FAQ):
We will make samples before mass production, and after sample approved, we’ll begin mass production. Doing 100% inspection during production, then do random inspection before packing.
Our MOQ is 1kg. But usually we accept less quantity such as 100g on the condition that sample charge is 100% paid.
Yes. We’ll give you product analysis report before shipping.
Different quantity has different discount.
Yes. Welcome to visit.
You can get free samples for some products,you only need to pay the shipping cost or arrange a courier to us and take the samples. You can send us your product specifications and requests,we will manufacture the products according to your requests.